Fate & Transport Assessment
Since mobility, persistence, and bioavailability are linked to chemical and physical properties, we employ advanced analytical techniques with standardized methods to quantify the state of nanomaterials in natural systems. Fate and transport studies determine how nanomaterials interact with natural matrices in order to predict their behavior in the event of an environmental release.
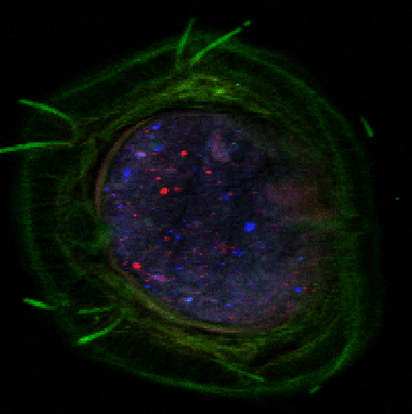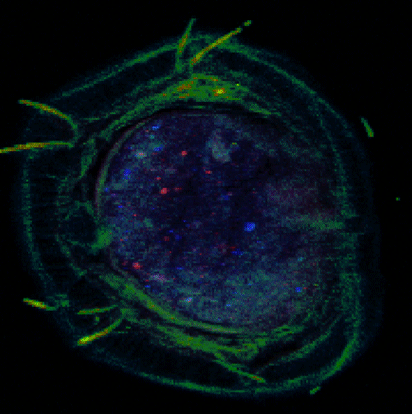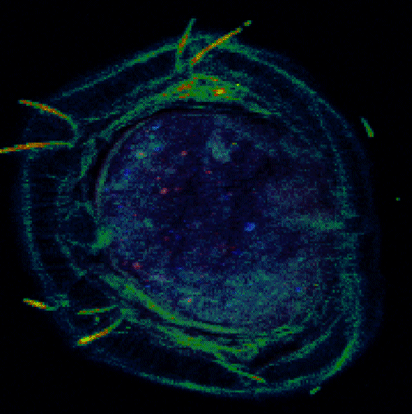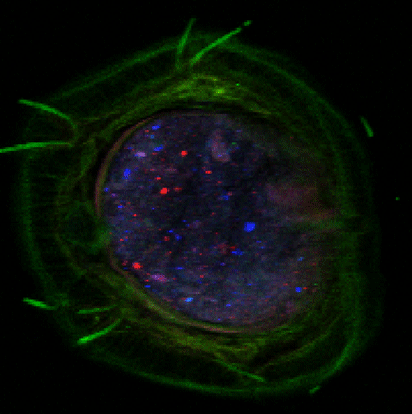
Advanced characterization, composition, and mapping of nanoparticles on solid phase samples is conducted using synchrotron-based techniques
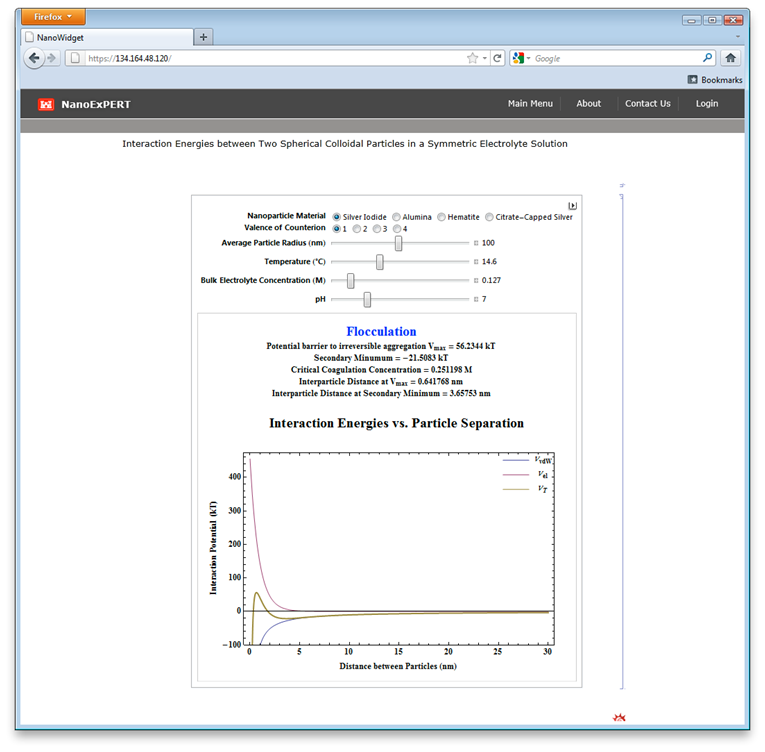
ERDC has developed mechanistic models to predict fate of nanoparticles in realistic environmental conditions
Capabilities
- Inductively Coupled Plasma Mass Spectrometry with Dynamic Reaction Cell interference reduction capability
- Laser Light Scattering
- Nuclear Magnetic Resonance
- Particle Charge (Zeta potential)
- Quartz Crystal Microbalance
Education
- Ph.D. Soil Science, 2004; Iowa State University; Ames, IA
- M.S. Plant & Soil Science, 1998; University of Kentucky; Lexington, KY
- B.S. Agronomy, 1995; Brigham Young University; Provo, UT
Research Interests
- Metal/Metalloid speciation in coal-combustion fly ash after prolonged submersion in a natural river system
- Quantity-Intensity nutrient ion relationships in the soil residence times of munition constituents
- Nanoparticle storage of cavitation-generated radical species
- Transport of B. subtilis spores through environmental and anthropogenic porous media
Education
- B.S. Biology, 1987; Texas A&M University; College Station, TX
Research Interests
- Mobility of nanoparticles in soils and sediments
- Dissolution and surface reactivity of nanoparticles in natural systems
- Stability of nanomaterials under environmental conditions
- Expertise in volatile, semi-volatile, and particulate emissions of organic and inorganic compounds from soils, sediments, and aqueous systems